Methanol
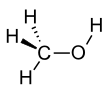
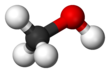
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic alcohol, with the chemical formula CH3OH (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, volatile, colorless and flammable liquid with a distinctive alcoholic odor similar to that of ethanol (potable alcohol), but is more acutely toxic than the latter.[17] Methanol acquired the name wood alcohol because it was once produced through destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide.[18]
Enquiry Form
Product Info
|
|||
 |
|||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Methanol[1]
|
|||
| Other names
Carbinol
Columbian spirits Hydroxymethane MeOH Methyl alcohol Methyl hydrate Methyl hydroxide Methylic alcohol Methylol Methylene hydrate, primary alcohol Pyroligneous spirit Wood alcohol Wood naphtha Wood spirit |
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| 1098229 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.599 | ||
| EC Number |
|
||
| 449 | |||
| KEGG | |||
| MeSH | Methanol | ||
|
PubChem CID
|
|||
| RTECS number |
|
||
| UNII | |||
| UN number | 1230 | ||
|
CompTox Dashboard (EPA)
|
|||
| Properties | |||
| CH3OH | |||
| Molar mass | 32.042 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | Faint and similar to ethanol | ||
| Density | 0.792 g/cm3[2] | ||
| Melting point | −97.6 °C (−143.7 °F; 175.6 K) | ||
| Boiling point | 64.7 °C (148.5 °F; 337.8 K) | ||
| miscible | |||
| log P | −0.69 | ||
| Vapor pressure | 13.02 kPa (at 20 °C) | ||
| Acidity (pKa) | 15.5[3] | ||
| Conjugate acid | Methyloxonium[4] | ||
| Conjugate base | Methanolate[5] | ||
| −21.40·10−6 cm3/mol | |||
|
Refractive index (nD)
|
1.33141[6] | ||
| Viscosity | 0.545 mPa·s (at 25 °C)[7] | ||
| 1.69 D | |||
| Thermochemistry | |||
| 725.7 kJ/mol, 173.4 kcal/mol, 5.77 kcal/g | |||
| Hazards[12][13] | |||
| Occupational safety and health (OHS/OSH): | |||
|
Main hazards
|
Methanol and its vapours are flammable.Moderately toxic for small animals – Highly toxic to large animals and humans (in high concentrations) – May be fatal/lethal or cause blindness and damage to the liver, kidneys, and heart if swallowed – Toxicity effects from repeated over exposure have an accumulative effect on the central nervous system, especially the optic nerve – Symptoms may be delayed, become severe after 12 to 18 hours, and linger for several days after exposure[9] | ||
| GHS labelling: | |||
   [8] [8] |
|||
| Danger[8] | |||
| H225, H301, H302, H305, H311, H331, H370[8] | |||
| P210, P233, P235, P240, P241, P242, P243, P260, P264, P270, P271, P280, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P307+P311, P310, P311, P312, P337+P313, P361, P363, P370+P378, P403+P233, P405, P501[8] | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 11 to 12 °C (52 to 54 °F; 284 to 285 K) | ||
| 470 °C (878 °F; 743 K)[15] 385 °C (725 °F; 658 K)[16] |
|||
| Explosive limits | 6–36%[10] | ||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
5628 mg/kg (rat, oral) 7300 mg/kg (mouse, oral) 12880 mg/kg (rat, oral) 14200 mg/kg (rabbit, oral)[11] |
||
|
LC50 (median concentration)
|
64,000 ppm (rat, 4 h)[11] | ||
|
LCLo (lowest published)
|
33,082 ppm (cat, 6 h) 37,594 ppm (mouse, 2 h)[11] |
||
| NIOSH (US health exposure limits): | |||
|
PEL (Permissible)
|
TWA 200 ppm (260 mg/m3)[10] | ||
|
REL (Recommended)
|
TWA 200 ppm (260 mg/m3) ST 250 ppm (325 mg/m3) [skin][10] | ||
|
IDLH (Immediate danger)
|
6000 ppm[10] | ||
| Safety data sheet (SDS) | [1] | ||
| Related compounds | |||
|
Related compounds
|
Methanethiol Silanol Ethanol |
||







Reviews