Isopropyl alcohol
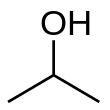
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable, organic compound with a pungent alcoholic odor.
Isopropyl alcohol, an organic polar molecule, is miscible in water, ethanol, and chloroform, demonstrating its ability to dissolve a wide range of substances including ethyl cellulose, polyvinyl butyral, oils, alkaloids, and natural resins. Notably, it is not miscible with salt solutions and can be separated by adding sodium chloride in a process known as salting out. It forms an azeotrope with water, resulting in a boiling point of 80.37 °C and is characterized by its slightly bitter taste. Isopropyl alcohol becomes viscous at lower temperatures, freezing at −89.5 °C, and has significant ultraviolet-visible absorbance at 205 nm. Chemically, it can be oxidized to acetone or undergo various reactions to form compounds like isopropoxides or aluminium isopropoxide. As an isopropyl group linked to a hydroxyl group (chemical formula (CH3)2CHOH) it is the simplest example of a secondary alcohol, where the alcohol carbon atom is attached to two other carbon atoms. It is a structural isomer of propan-1-ol and ethyl methyl ether. They all have the formula C3H8O.
Enquiry Form
Product Info
|
|||
 |
|||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Propan-2-ol[2]
|
|||
| Other names
2-Propanol
Isopropanol[1] Rubbing alcohol sec-Propyl alcohol 2-Hydroxypropane i-PrOH Dimethyl carbinol IPA |
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| 635639 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.601 | ||
| 1464 | |||
| KEGG | |||
|
PubChem CID
|
|||
| RTECS number |
|
||
| UNII | |||
| UN number | 1219 | ||
|
CompTox Dashboard (EPA)
|
|||
| Properties | |||
| C3H8O | |||
| Molar mass | 60.096 g/mol | ||
| Appearance | Colorless liquid | ||
| Odor | Pungent alcoholic odor | ||
| Density | 0.786 g/cm3 (20 °C) | ||
| Melting point | −89 °C (−128 °F; 184 K) | ||
| Boiling point | 82.6 °C (180.7 °F; 355.8 K) | ||
| Miscible with water | |||
| Solubility | Miscible with benzene, chloroform, ethanol, diethyl ether, glycerol; soluble in acetone | ||
| log P | −0.16[3] | ||
| Acidity (pKa) | 16.5[4] | ||
| −45.794·10−6 cm3/mol | |||
|
Refractive index (nD)
|
1.3776 | ||
| Viscosity | 2.86 cP at 15 °C 1.96 cP at 25 °C[5] 1.77 cP at 30 °C[5] |
||
| 1.66 D (gas) | |||
| Pharmacology | |||
| D08AX05 (WHO) | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
|
Main hazards
|
Flammable, mildly toxic[6] | ||
| GHS labelling: | |||
  |
|||
| Danger | |||
| H225, H302, H319, H336 | |||
| P210, P261, P305+P351+P338 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | Open cup: 11.7 °C (53.1 °F; 284.8 K) Closed cup: 13 °C (55 °F) |
||
| 399 °C (750 °F; 672 K) | |||
| Explosive limits | 2–12.7% | ||
|
Threshold limit value (TLV)
|
980 mg/m3 (TWA), 1225 mg/m3 (STEL) | ||
| Lethal dose or concentration (LD, LC): | |||
|
LD50 (median dose)
|
|||
|
LC50 (median concentration)
|
|
||
|
LCLo (lowest published)
|
|
||
| NIOSH (US health exposure limits): | |||
|
PEL (Permissible)
|
TWA 400 ppm (980 mg/m3)[7] | ||
|
REL (Recommended)
|
TWA 400 ppm (980 mg/m3), ST 500 ppm (1225 mg/m3)[7] | ||
|
IDLH (Immediate danger)
|
2000 ppm[7] | ||
| Safety data sheet (SDS) | [1] | ||
| Related compounds | |||
|
Related alcohols
|
1-Propanol, ethanol, 2-butanol | ||
| Supplementary data page | |||
| Isopropyl alcohol (data page) | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||







Reviews