Ammonium Nitrate
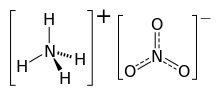
Ammonium nitrate is a chemical compound with the formula NH4NO3. It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, but does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer.
Its other major use is as a component of explosive mixtures used in mining, quarrying, and civil construction. It is the major constituent of ANFO, an industrial explosive which accounts for 80% of explosives used in North America; similar formulations have been used in improvised explosive devices.
Enquiry Form
Product Info
 |
|
 |
|
 |
|
| Names | |
|---|---|
| IUPAC name
Ammonium nitrate
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.026.680 |
| EC Number |
|
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 0222 – with > 0.2% combustible substances 1942 – with ≤ 0.2% combustible substances 2067 – fertilizers 2426 – liquid |
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| NH4NO3 | |
| Molar mass | 80.043 g/mol |
| Appearance | white crystalline solid |
| Density | 1.725 g/cm3 (20 °C) |
| Melting point | 169.6 °C (337.3 °F; 442.8 K) |
| Boiling point | approx. 210 °C (410 °F; 483 K) decomposes |
| Endothermic 118 g/100 ml (0 °C) 150 g/100 ml (10 °C) 192 g/100 ml (20 °C) 297 g/100 ml (40 °C) 410 g/100 ml (60 °C) 576 g/100 ml (80 °C) 1024 g/100 ml (100 °C)[1] |
|
| −33.6×10−6 cm3/mol | |
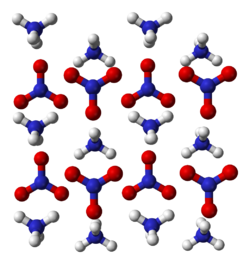
| Structure | |
| orthorhombic[2] | |
| Explosive data | |
| Shock sensitivity | very low |
| Friction sensitivity | very low |
| Detonation velocity | 2500 m/s |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
|
Main hazards
|
Explosive, Oxidizer |
| GHS labelling: | |
   |
|
| Danger | |
| H201, H271, H319 | |
| P220, P221, P264, P271, P280, P372 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
2085–5300 mg/kg (oral in rats, mice)[3] |
| Related compounds | |
|
Other anions
|
Ammonium nitrite |
|
Other cations
|
Sodium nitrate Potassium nitrate Hydroxylammonium nitrate |
|
Related compounds
|
Ammonium perchlorate |







Reviews